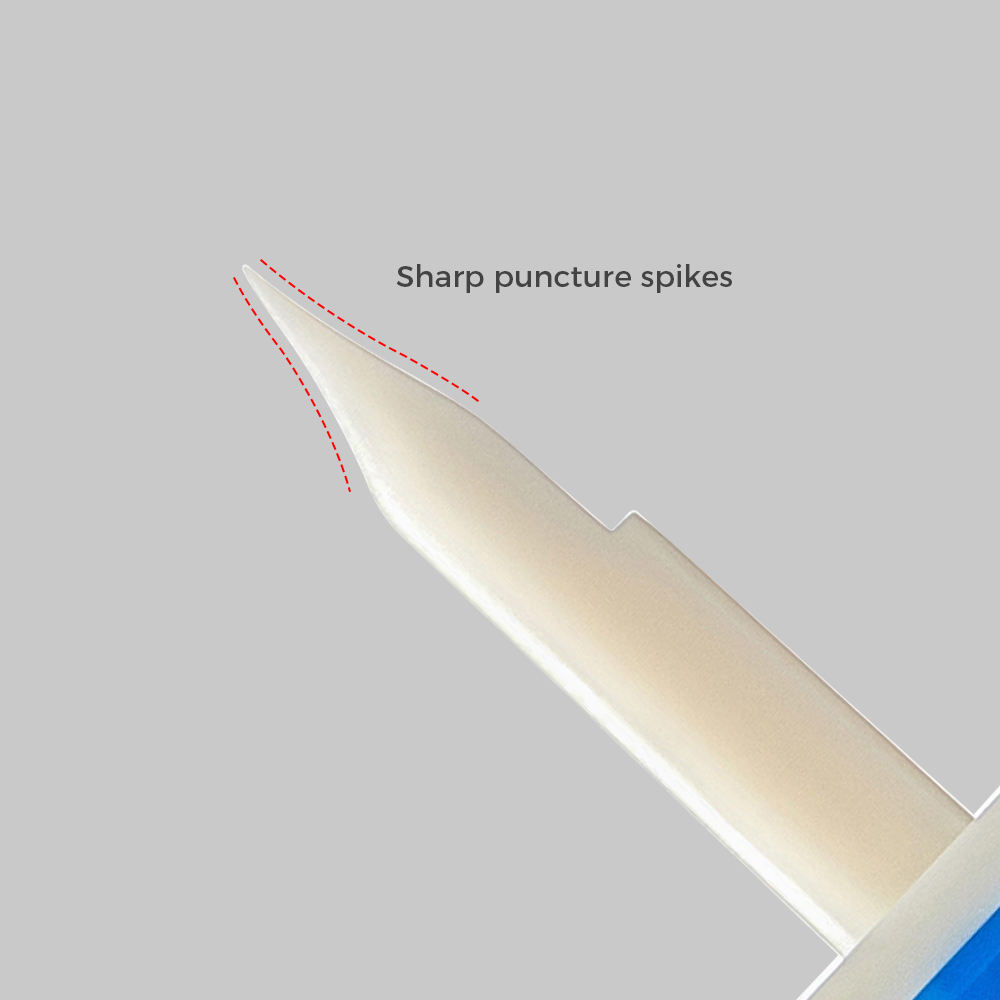
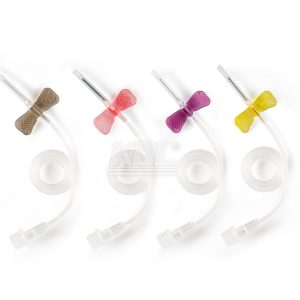
Withdrawal and Injection Spikes
● Sterile, Non-Toxic, Non-Pyrogenic
● Complete the transfer of liquid between two containers
● Provide a sterile environment for medicinal solutions
● Reduce contamination during drug transfer
ltem No. : 4488Product Features
| Intended use | The product is designed to transfer medical fluids between a first container (s) [e.g. a vial (s)] and a second container [e.g. an intravenous (IV) bag] it is not dedicated to a particular type of fluid or clinical procedure. |
| Structure and compostion | Consists of spike, protective cap for spike and filter for female conical fitting, air cap (Optional), folding cap (Optional), needle-free connector (Optional), filter membrane of air (Optional), filter membrane of liquid (Optional) |
| Shelf life | 5 years |
| Certification and Quality Assurance | In compliance with REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL (CE Class: Is) Manufacturing process is in compliance with ISO 13485 Quality System. |
Main Material
| Spike | ABS, MABS |
| Filter for female conical fitting | MABS |
| Air cap | MABS |
| Protect cap for spike | MABS |
| Folding cap | PE |
| Rubber plug | TPE |
| Valve plug | MABS |
| Needle-free connector | PC+Silicone rubber |
| Adhesive | Light-curing adhesives |
| Pigment (Folding cap) | Blue/Green |
| Filter membrane of air | PTFE 0.2μm/0.3μm/0.4μm |
| Filter membrane of liquid | PES 5μm/3μm/2μm/1.2μm |
 +86-791-8686-1216
+86-791-8686-1216